Hole in your heart? Leave it.
The story of how a $10,000 umbrella failed to significantly reduce stroke risk.
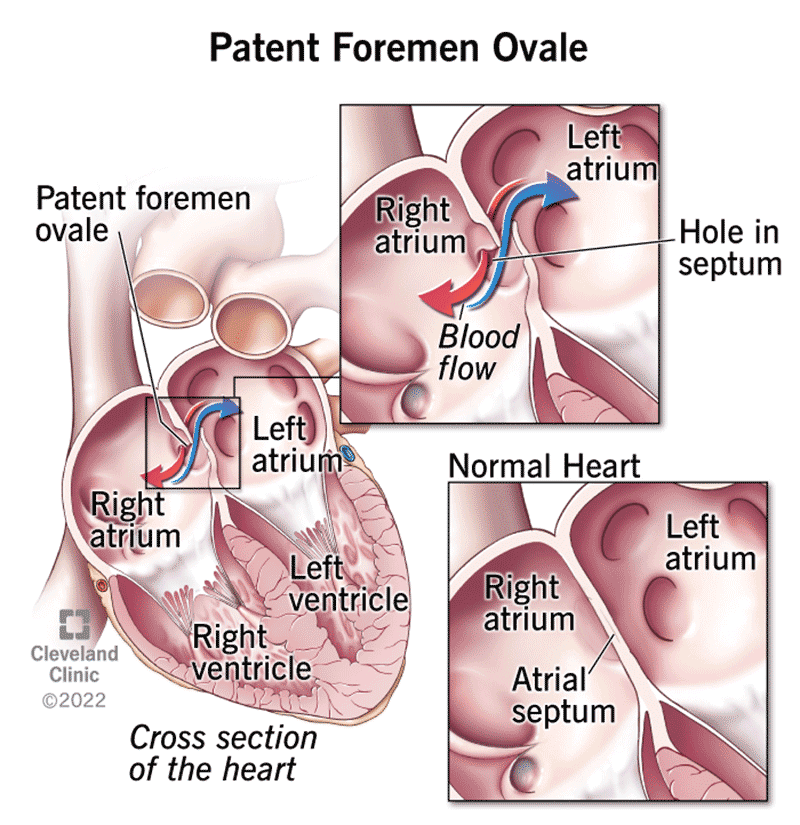
Patent Foramen Ovale (and why we care)
Before you’re born, your blood doesn’t really need to go to the lungs to be oxygenated– placenta takes care of that. There are a number of temporary bypasses in utero that allow oxygenated blood to come in, circulate, and be pumped back out to the placenta. After birth, the fluid from the lungs empties and there’s a massive reduction in pressure, forcing these temporary bypass circuits to close– in approximately 25% of adults, a small hole between the circuits remains.
For most adults, you’d never know this is an issue, very little blood can pass through this small space. However, small clots that would normally be stopped in the lungs have a fast-track to the brain to cause an unexpected stroke (Cryptogenic Stroke). Strokes in patients under age 60, of unknown cause made up about 25% of all ischemic strokes, and PFOs can 3x the risk of stroke.
For the 16,000 adults with a PFO, who suffer from a cryptogenic stroke before age 55, the only real treatment before the advent of occluder devices was anticoagulation. Sounds simple enough– if you stay on enough blood thinner, you’re less likely to form any clots and thus, less likely to have another stroke.
However, blood thinners in young adults are an imperfect treatment. For one, you have to consistently take them, which literally any human behavior researcher will tell you is unlikely to happen over the course of decades. Two, if you happen to be in center of the venn-diagram of clumsy and stroke-prone, blood thinners could lead to some nasty bleeds. But most of all, even on high-dose aspirin, your risk of stroke is not necessarily significantly reduced.
Humanitarian Device Exemptions
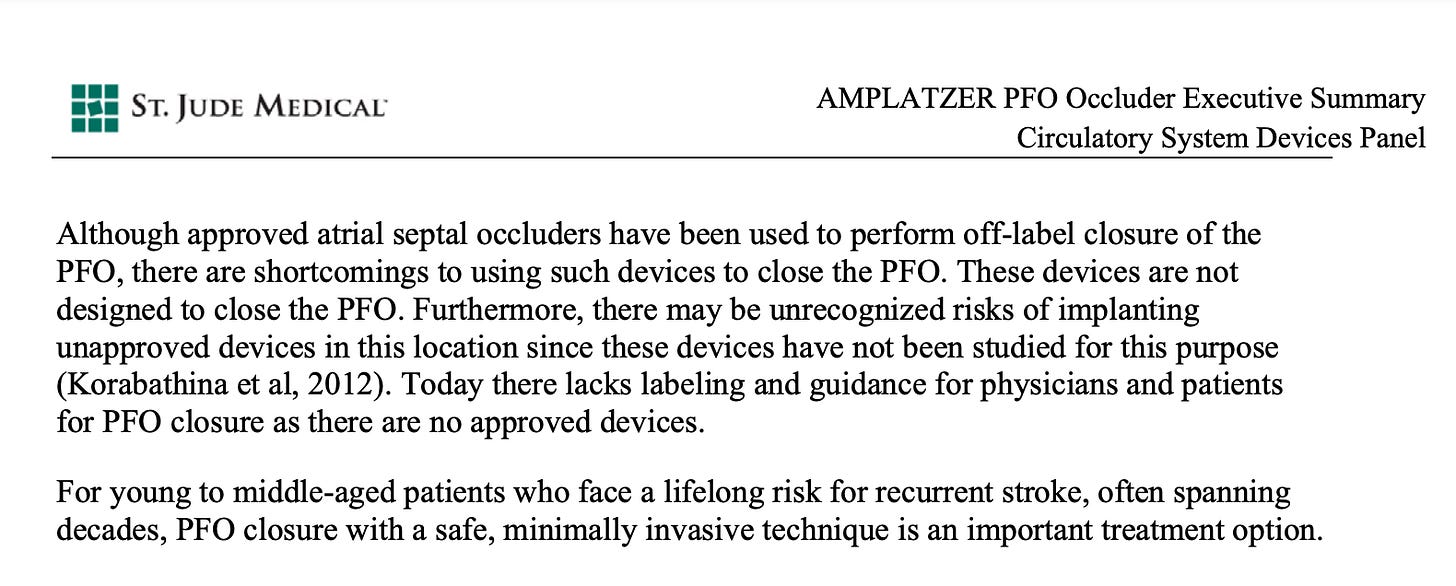
In 2000, St. Jude Medical Inc.’s Amplatzer PFO Occluder started the super-straightforward process of seeking FDA approval for a PFO-specific closure device, the first in its class, via a PFO Phase I Feasibility study. (Note: there were other devices used off-label for PFO closure before this, and some interesting surgical techniques, but we’ll gloss over those).
PFO devices were used in 79 patients who had experienced cryptogenic stroke/TIA (mini-stroke, basically), or peripheral embolism. The “Unmet Need” Section of the FDA application outlines that these devices were intended for a very specific niche: young, healthy adults who had previously suffered an unexpected stroke and would be better managed with a device for stroke prevention, rather than lifelong anticoagulation.
In 2002 the feasibility study was completed, and the RESPECT Trial was set to begin enrolling.
Given that these devices were primarily meant for a very small population of young and middle aged adults, the FDA allowed for these devices to go to market under Humanitarian Device Exemption, rather than wait for the results of the RESPECT Trial. This exemption allowed for up to 4,000 patients per year to be eligible while the clinical trial was ongoing.
Between 2002-2006, consistently more Amplatzer PFO occluders were shipped than the FDA permitted HDE limit, and the HDE-approval was withdrawn. Its theorized that the FDA did this to increase enrollment in the RESPECT Trial, however, this did not appear to improve trial recruitment and with no restrictions on the off-label use of these devices pre-approval, set off a frenzy. The manufacturer created the PFO ACCESS IDE Registry, a registry for patients using the device under Investigational Device Exemption, and thus allowed physicians to use their own discretion with off-label device use.
Industry never revealed just how many devices were placed, but by the time the second clinical trial (CLOSURE) began enrolling to assess outcomes from these devices, there were 7 in the market being used off-label.
These devices billed about $10,000 for a 15 minute procedure and became extremely lucrative. With 95% of procedures being successful, it was way too easy to start putting these in everyone.
There were fairly hand-wavey requirements around neurologic imaging or around the failure of anticoagulation required before placement. Devices started being placed in young adults with a PFO without history of stroke, or necessarily failure of anticoagulation– or they must have been, given the estimates on number of devices exceed the number of estimated eligible patients under initial Phase I eligibility.
Off-label uses such as PFO closure for “migraine” or “decompression sickness” in performance-athlete divers emerged– with no clinical trial data to back either these devices efficacy in primary prevention of stroke, or any real evidence they would actually treat migraines or decompression sickness.
Side note: For the single ultra-marathoner reading this, no, you should not bully your cardiologist into placing one of these to increase your VO2 max.
The Results
Ultimately, in March 2012 the results of the 909 patients enrolled in the CLOSURE Trial were presented, with the RESPECT Trial presenting shortly after.
Patients enrolled had a history of cryptogenic stroke, and received either percutaneous PFO Closure (STARFlex Device) and then anti-platelet therapy after the procedure (as is standard), or medication-based anticoagulation (warfarin, aspirin, or both).
There were no significant differences in risk of stroke/TIA, death from any cause, or death from neurologic causes between the two groups.
The RESPECT Trial reached the same conclusions, despite TIA (mini-stroke) being excluded from enrollment in the trial.
Conclusion
It took nearly 13 years from the initial approval of PFO closure devices to the publication of multi-specialty guidelines outlining the appropriate use for select populations. Thousands, if not hundreds of thousands, of devices were placed inappropriately in the interim with almost no evidence to stand on, due to the FDA’s lack of requirement for adequate clinical trial data prior to approval.